Abstract
INTRODUCTON: The Guideline for Prescribing Opioids for Chronic Pain instituted by the Centers for Disease Control and Prevention (CDC) in 2016 has been found to be effective in decreasing opioid use. However, this decrease has included patient groups that fall outside of the intended scope of the guideline such as patients with sickle cell disease (SCD) who already experience difficulty accessing needed pain management. The aim of this study was to evaluate the effect of CDC's 2016 Opioid Guideline on opioid prescribing level and health outcomes among patients with SCD. Because patients with SCD may experience different symptoms and complications as they age, the consequences were assessed by age group.
METHODS: This retrospective cohort study employed an interrupted time series analysis using the IBM® MarketScan® Commercial Database from 1/1/2011 to 12/31/2019. The study population included individuals ≥1 years old who had ≥3 SCD diagnosis codes within 5 years and had no cancer diagnosis. Monthly-measured opioid prescribing levels included: opioid prescription rate, mean daily morphine milligram equivalents (MME) per prescription, and mean number of days supplied per prescription. Health outcomes included: monthly rates of vaso-occlusive crisis (VOC)-related emergency department (ED) visits and hospitalizations, and monthly rates of healthcare facility visits related to opioid use disorder (OUD), depression, and anxiety. Segmented regressions (breakpoint: March 2016) were conducted for all outcomes to compare trends before and after the guideline release.
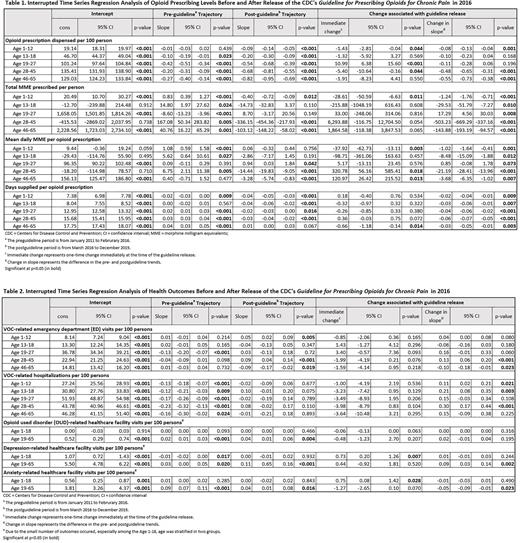
RESULTS: The study included 14,979 SCD patients (1-65 years old, mean [SD] age = 25.9 [16.9], 56.9% female). After the guideline release, compared to the pre-guideline period, 1) children (1-12 years; N=3,916 [26.4%]) experienced significant decreases in the opioid prescription rate (-0.09 prescription/100 person-month, p=0.001), mean daily MME per prescription (-1.02 MME/month, p=0.001) and the number of days supplied per prescription (-0.02 days/month, p=0.009), but a significant increase in the VOC-related hospitalization rate (+0.11 hospitalizations/100 person-month, p=0.021); 2) adolescents (13-18 years; N=1,782 [11.9%]) experienced significant decreases in the mean daily MME per prescription (-8.48 MME/month, p=0.012) and the number of days supplied (-0.03 days/month, p=0.003), but an increase in the VOC-related hospitalization rate (+0.21 hospitalizations/100 person-month, p=0.003); 3) young adults (19-27 years; N=2,599 [17.4%]) experienced a significant decrease in the number of days supply (-0.04 days/month, P<0.001), and no significant change in the VOC-related outcomes; 4) adults 28-45 years (N=4,293 [28.7%]) experienced significant decreases in opioid prescription rate (-0.48 prescription/100 person-month, p<0.001), mean daily MME per prescription (-21.19 MME/month, p<0.001), and the number of days supplied (-0.06 days/month, p<0.001), but significant increases in VOC-related ED visit rate (+0.13 visits/month, p<0.001) and hospitalization rate (+0.30 hospitalizations/100 person-month, p<0.001); and 5) adults 46-65 years (N=2,344 [15.6%]) experienced significant decreases in the opioid prescription rate (-0.55 prescription/100 person-month, p<0.001), mean daily MME per prescription (-3.68 MME/month, p<0.001), the number of days supplied (-0.03 days/month, p=0.003), and VOC-related ED visit rate (-0.1 visits/100 person-months, p=0.023). OUD-related healthcare facility visits did not differ in either pediatric (1-18 years) or adult (19-65 years) patients before vs. after the guideline release. Depression-related visits increased significantly (+0.09 visits/100 person-months, p=0.002) among adult patients while anxiety-related visits decreased significantly (-0.05 visits/100 person-months, p=0.023) among the same population post-guideline compared to the pre-guideline periods.
CONCLUSIONS: The CDC guideline release was associated with a decrease in opioid prescribing levels across all age groups even though the guideline was intended only for the adult population. Overall, pain-related health outcomes were negatively impacted by the guideline: older age groups with increased ED visits and younger age groups with increased hospitalizations, while there were no decreases in the rate of OUD.
Disclosures
Ataga:Pfizer: Consultancy; Roche: Consultancy; Biomarin: Consultancy; Agios Pharmaceuticals: Consultancy; Forma Therapeutics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal